Quality management for the pharmaceutical industry
With our system, you will ensure the constant improvement of your company’s processes, as well as guarantee the quality of your products and services.
Aligned with the guidelines of RDC-301 – Good Manufacturing Practices for Medicines, from the National Health Surveillance Agency (ANVISA), four modules of the Interact Suite SA have been approved in quality management programs of laboratory sector clients in Brazil.
Benefits and features
- Compliance with RDC 301 guidelines – Good Drug Manufacturing Practices, by Anvisa
- Control and alignment of strategic and operational indicators
- Management tools for visual monitoring of indicators
- Control of action plans and projects with schedule and workflow
- Sophisticated management analysis tools
- Integrated management of critical analysis events
Want more benefits?
Register your informations so that we can contact you as soon as possible.
Request contactAvailable and complementary components

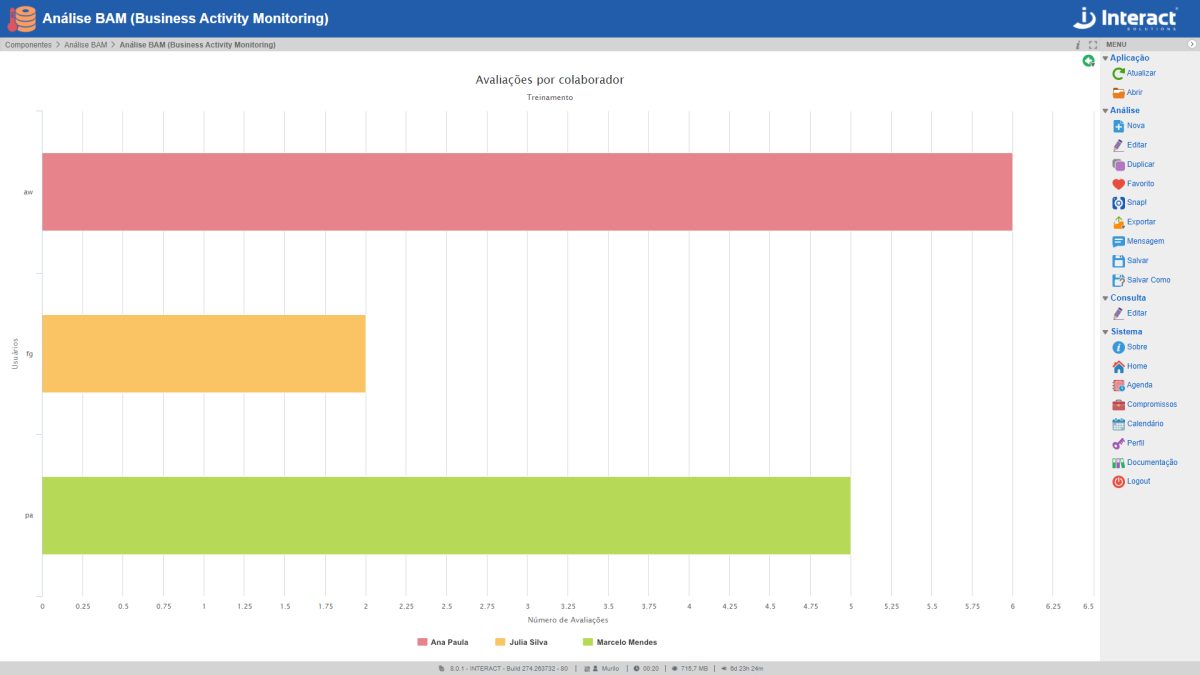
BAM Analysis
The SA BAM Analysis (Business Activity Monitoring) allows you to monitor the organization’s performance in real time, through interactive graphics. The Interact tool allows the selection of what data you want to view, with direct access to the database by Interact Suite SA. It’s all very dynamic. You update the chart data and it can be presented instantly, without the need to include it in a file in presentation format.


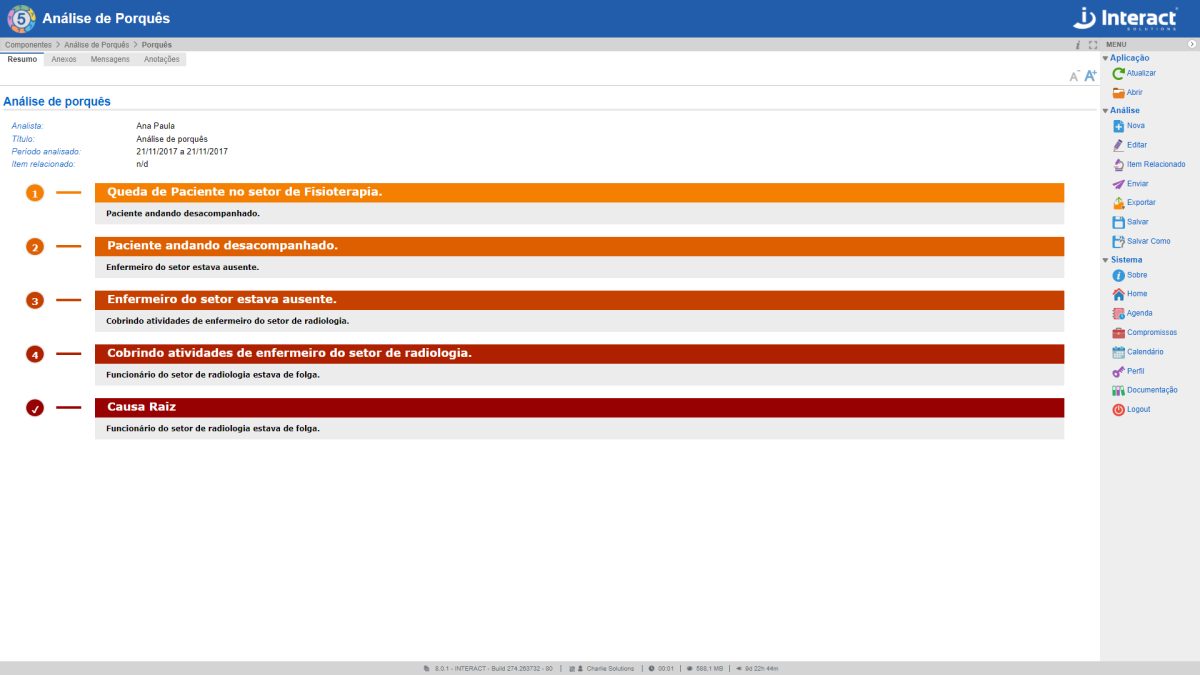
5 Whys
SA 5 Whys starts from the premise that, after asking 5 times the reason for the problem, it is possible to determine its root cause. This Suite SA tool makes it possible to perform this type of cyclic analysis, linked to the previous cause analyzed.


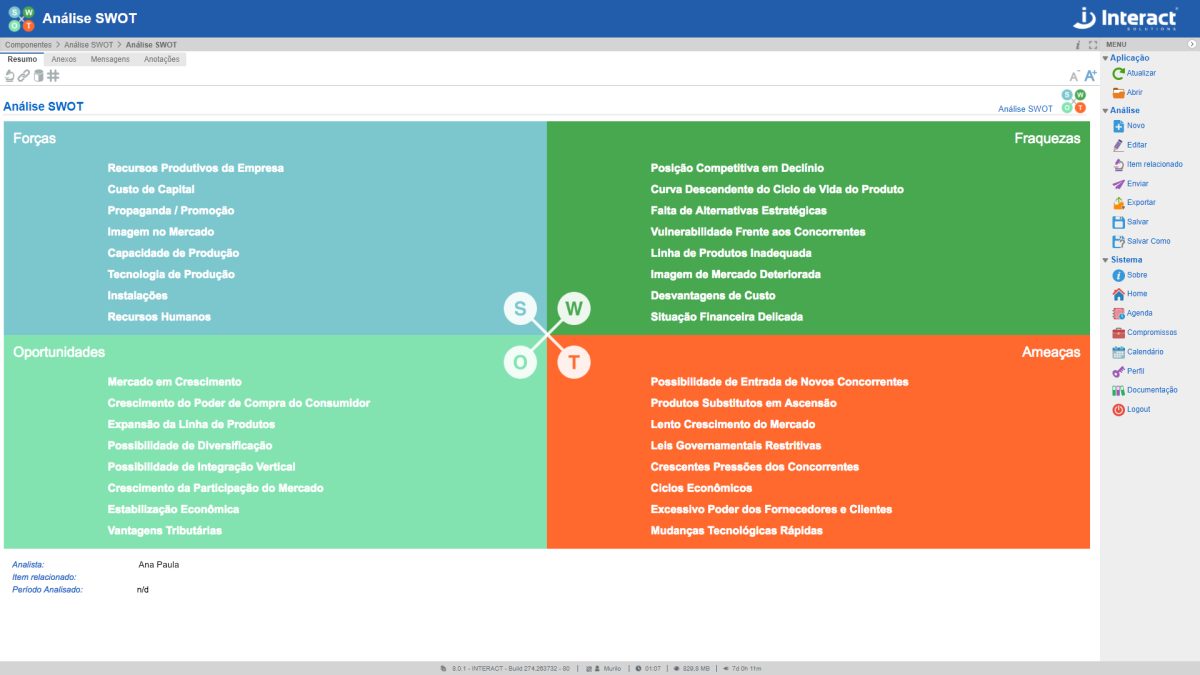
SWOT Analysis
SWOT Analysis (acronym for Strengths, Weaknesses, Opportunities and Threats) is a scenario or environment analysis tool, used as a basis for the management and strategic planning of an organization. Thanks to its simplicity, it can be used for any type of scenario analysis.


Presenter
Presenter is a tool for creating slide shows developed exclusively for Suite SA Strategic Adviser, with the possibility of links to indicators, projects and other elements of the Interact’s system.


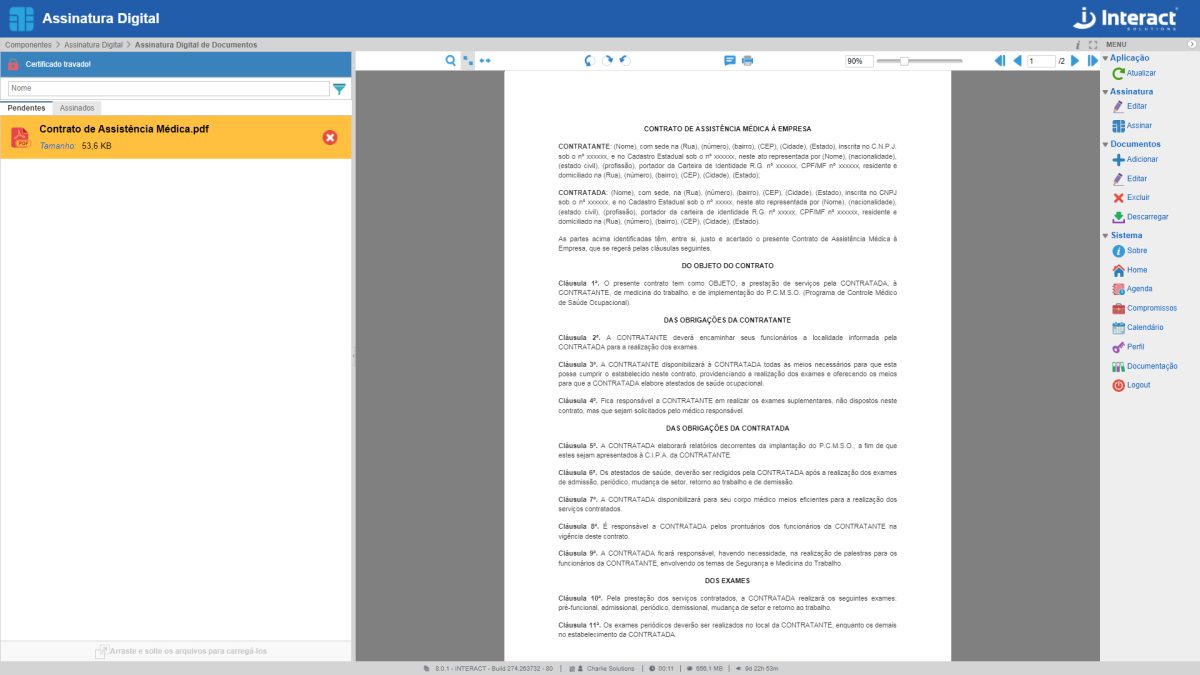
Digital Signature
The Digital Signature tool makes it possible to insert digital signatures in PDF files without having to go through the entire flow of documents, which speeds up the process. The type of certificate used in the documents is A1 and is also linked to the user. In addition, you can choose the type of signature as qualified or simplified.


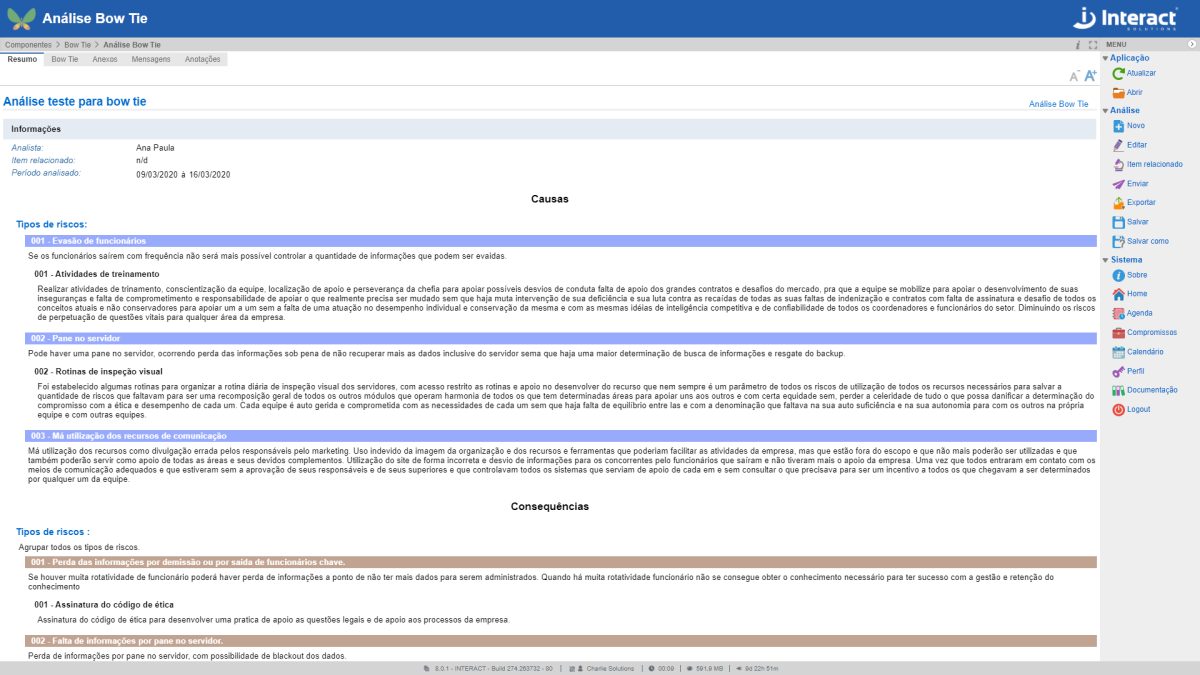
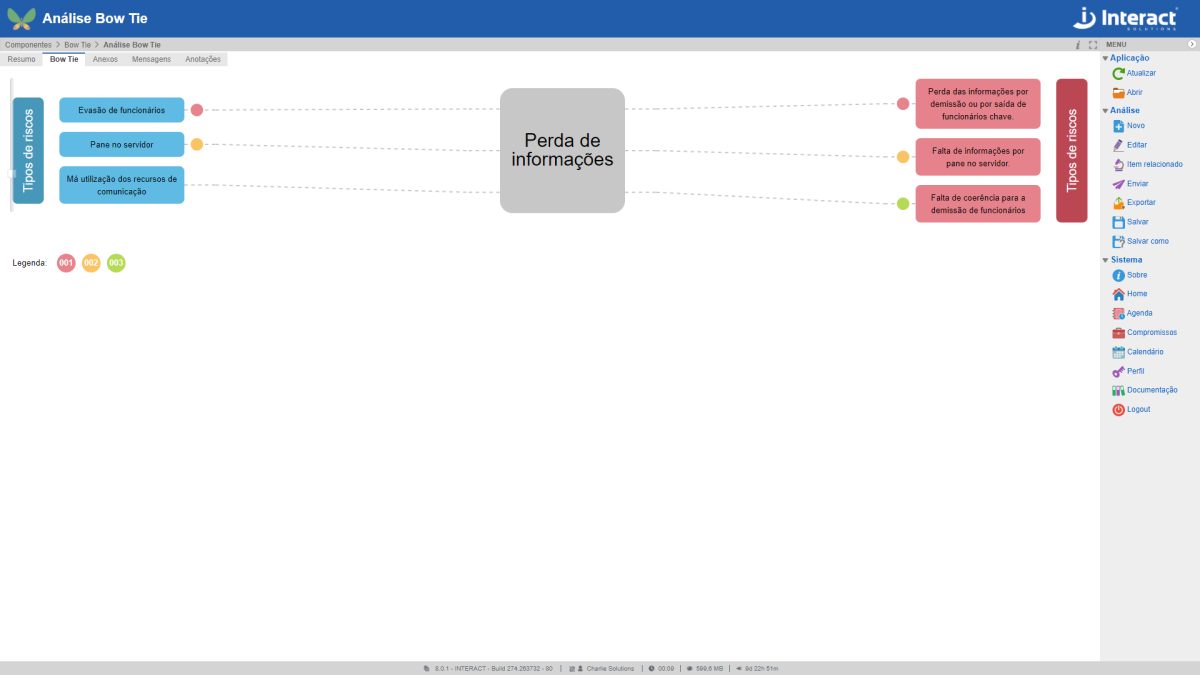
Bow Tie
Bow Tie analysis is a way of describing and analyzing a risk situation, examining the paths from causes to consequences. The focus of the Suite SA tool is on the barriers between causes and risk and between risk and consequences.


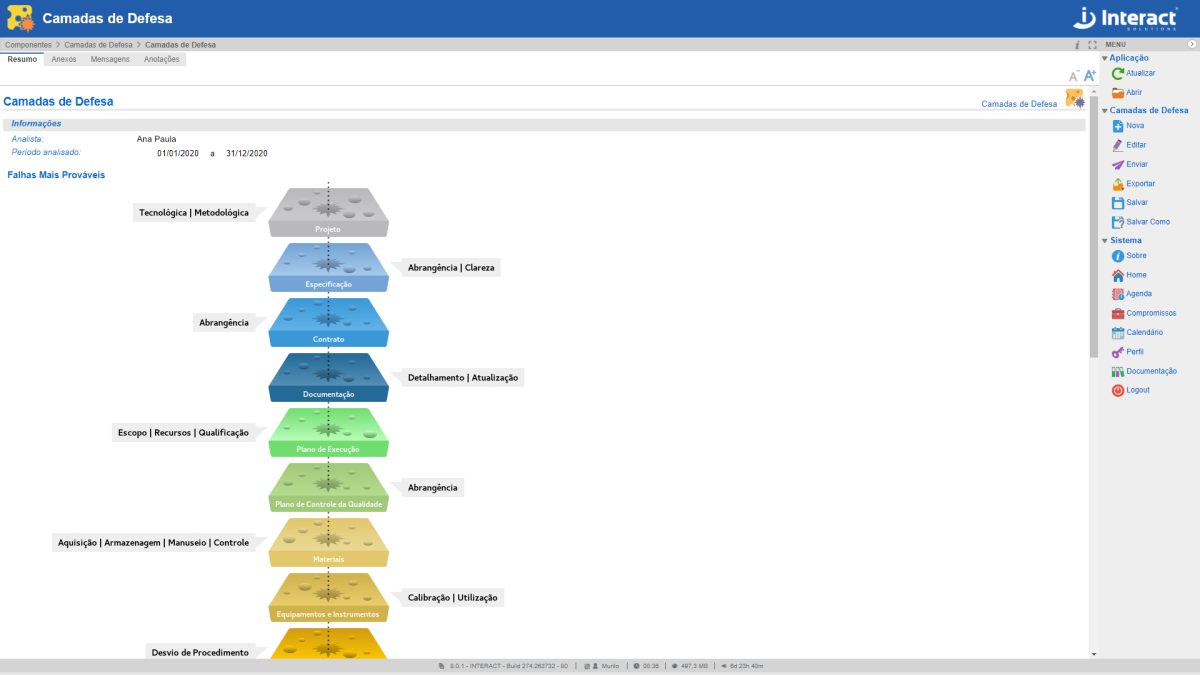
Defense Layers
Defense Layers are used to identify failures, dangers and possible losses in an organization. Each layer is represented as a slice of Swiss cheese, with holes considered as failures or hazards. After a sequence of failures (alignment of holes in the layers), it is possible that a loss will occur. This analysis by Suite SA offers a more improved risk management condition, with hole mitigation and reduction of failures in the organization.


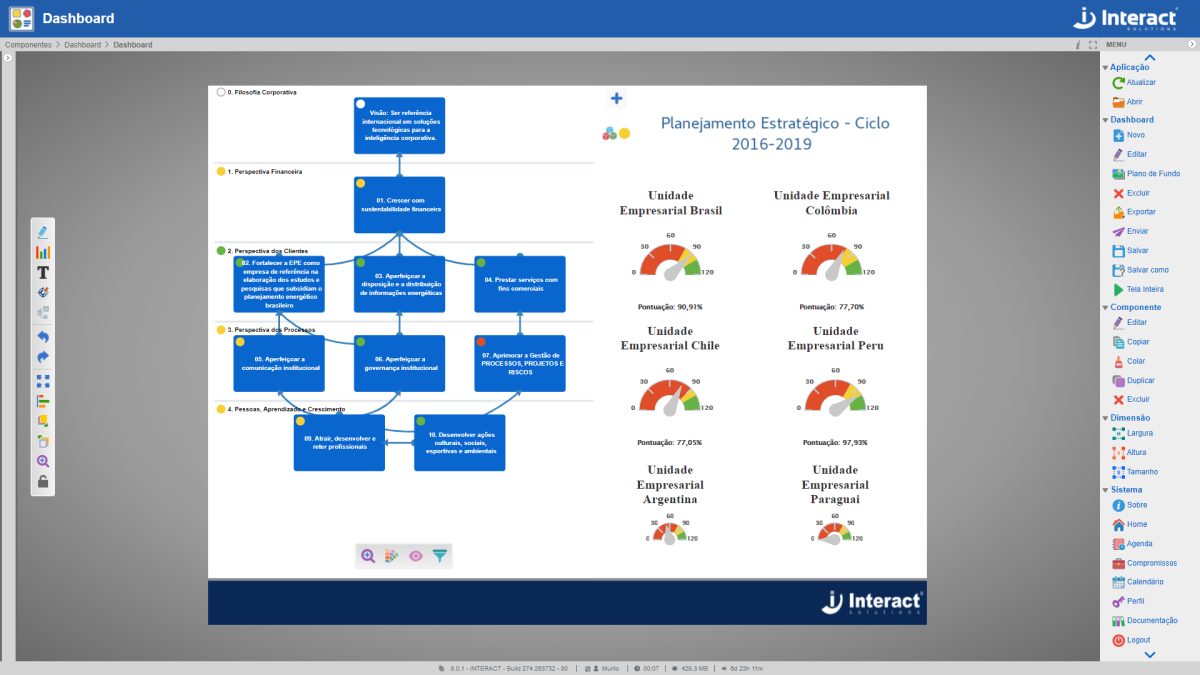
Dashboard
The Dashboard is a tool for strategic planning analysis, also used as a strategic map. With it, it is possible to create detailed organization charts and flow charts. It is a fundamental tool for organizations that use management methodologies or Balanced Scorecard (BSC).


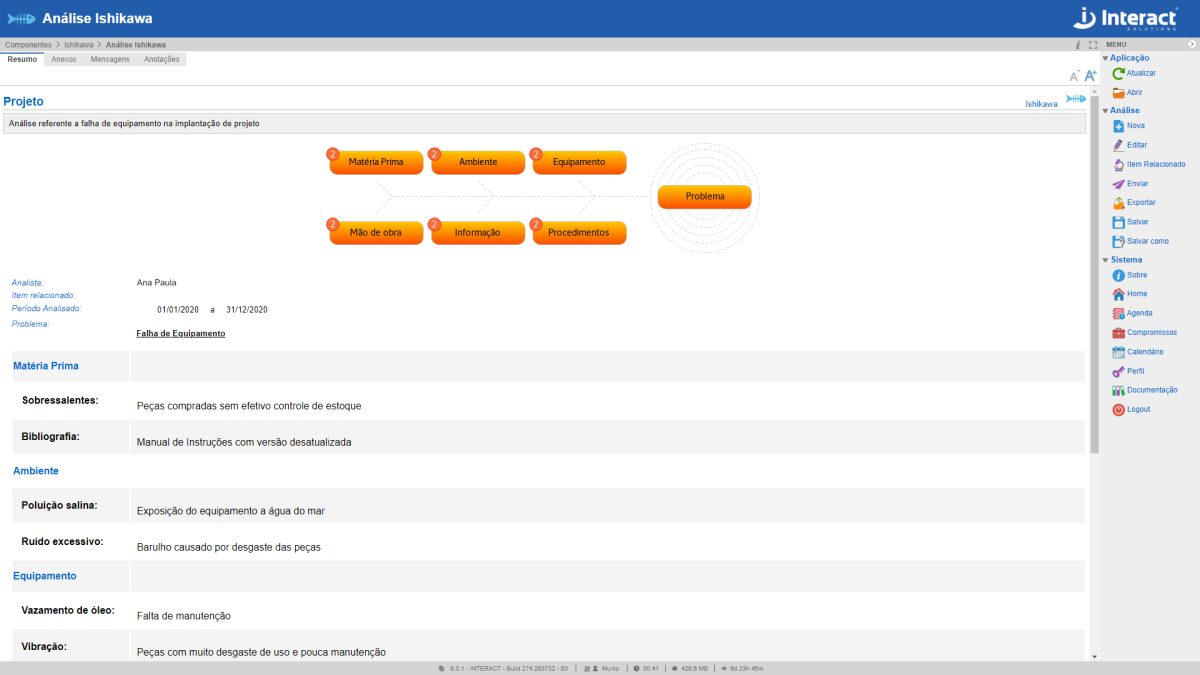
Ishikawa
The Ishikawa Diagram is a tool of Suite SA, used in improvement and quality control actions. It allows grouping and visualizing various causes of the origin of any problem or result that is to be improved.


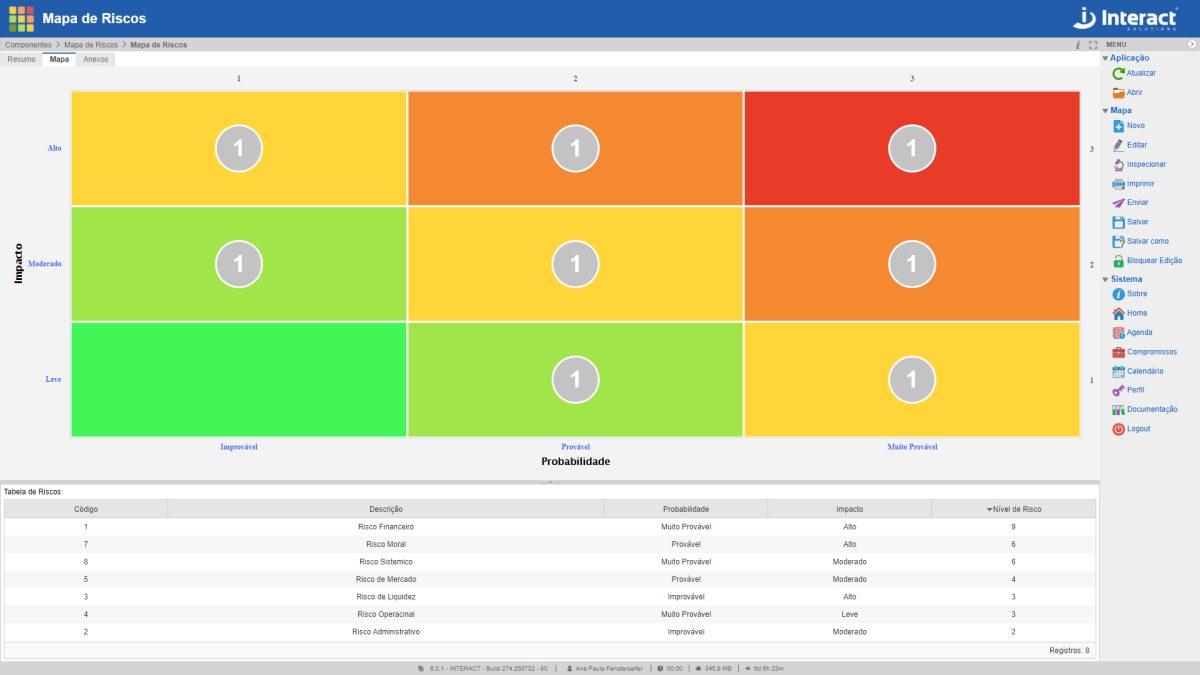
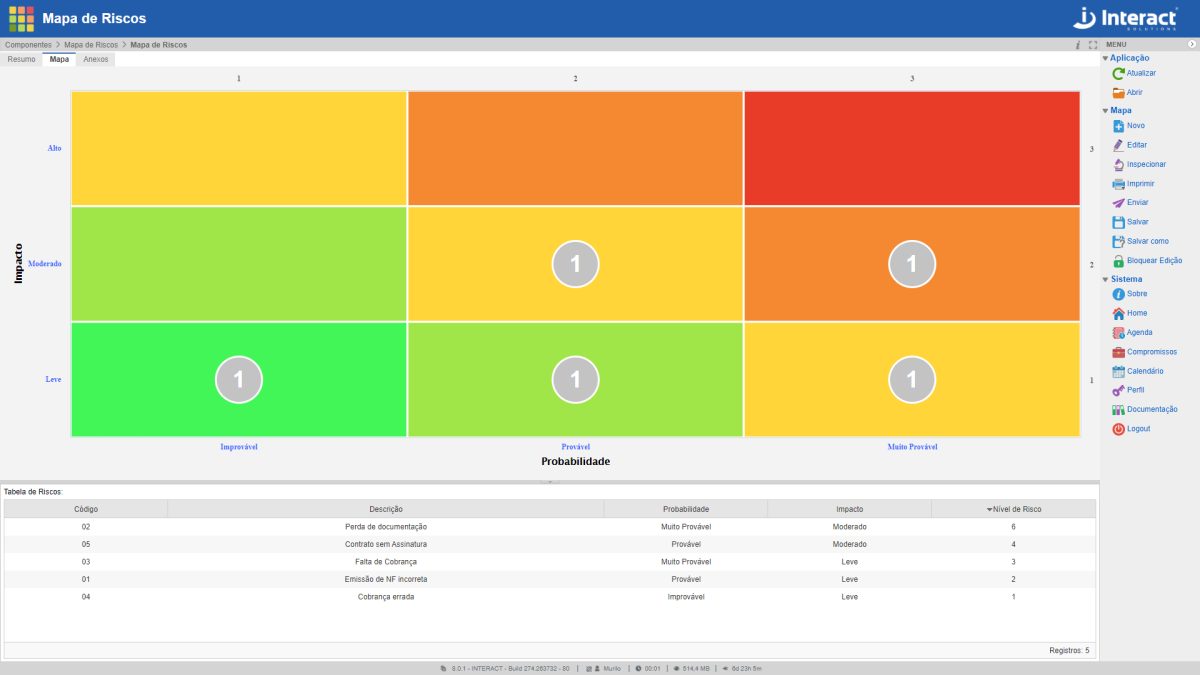
Risk Map
Risk Map is an Interact Suite SA tool for the graphical analysis of identified risks. It is a basic application, which allows the definition of elements that make up the current scenario, according to the organization’s Risk Management, with the definition of risks, impacts and probabilities. Risks are represented in the matrix by circles, which show the number of risks located in each quadrant cell, according to the determined colour.

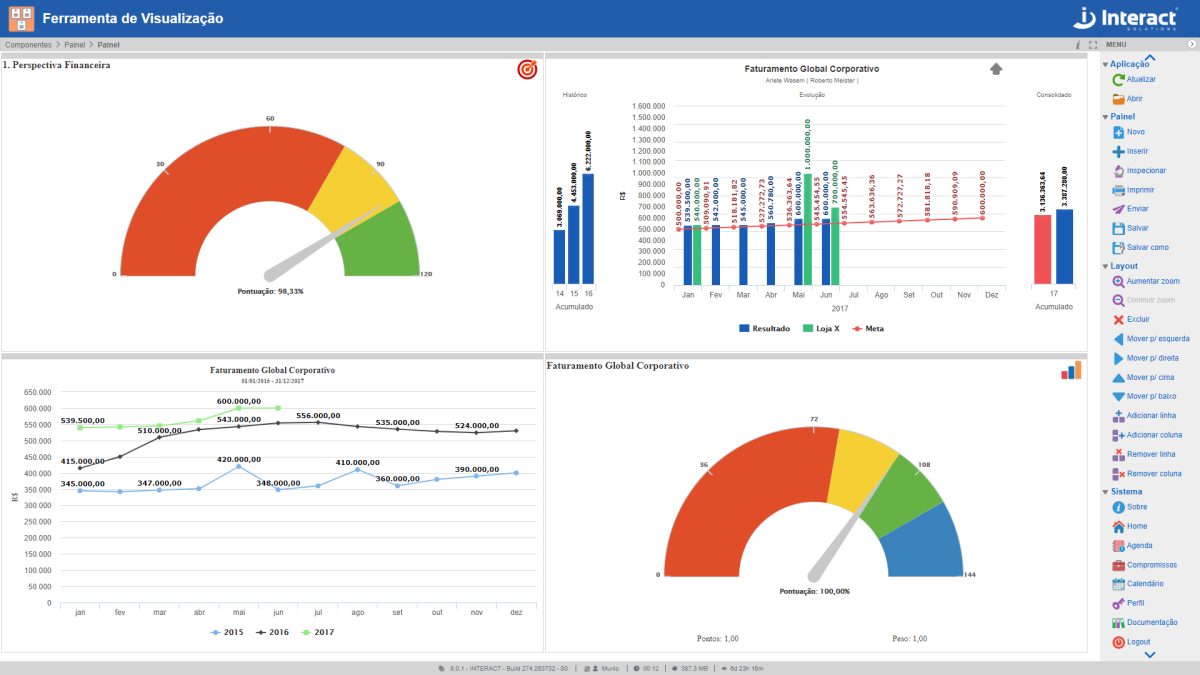
Panel
Panel de Suite SA presents exclusive tables to analyze organizational performance. In general, this tool could show performance indicators, action plans, comparative graphics or radars. In comparison, this is a better version of the department joints, from where they are added with results.


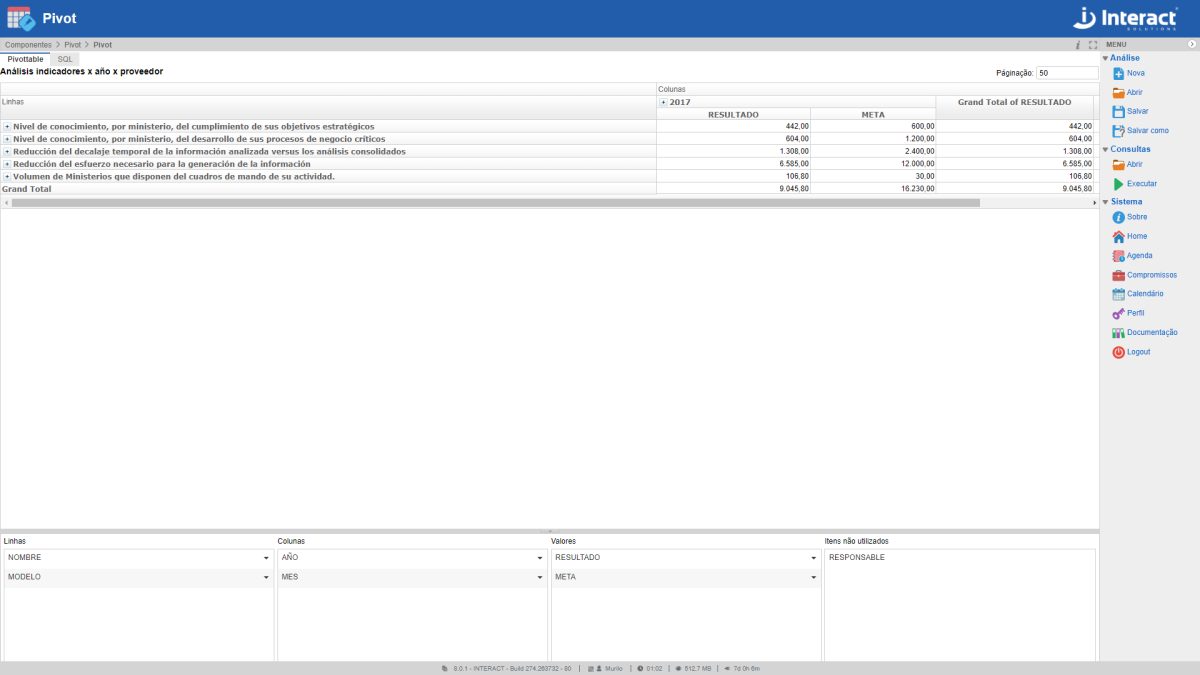
Pivot
The tool Pivot offers the user an efficient analysis of a large volume of data, with dynamic composition of tables, data set of different sources and filtering of relevant information.


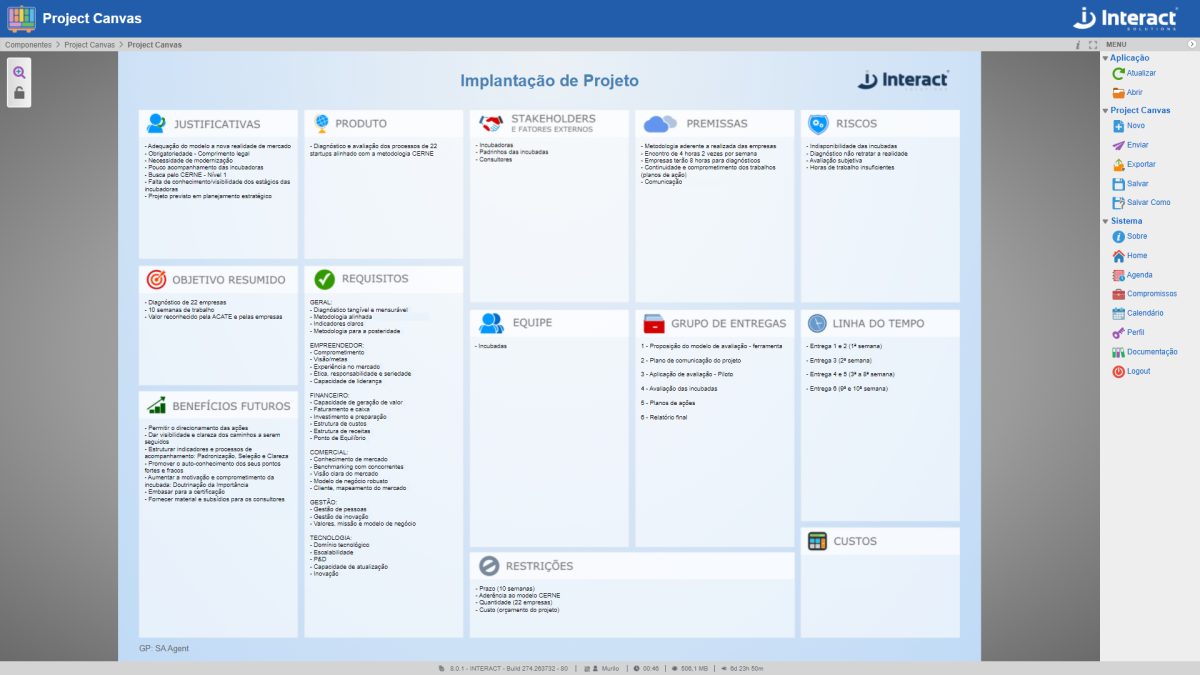
Project Canvas
Interact Project Canvas is a tool for developing the Project Model Canvas methodology, ideal for innovative and dynamic environments. Through it, it is possible to visualize the entire scope of a project in a single image and develop projects collaboratively. In addition, the application allows export in PDF and PNG formats.


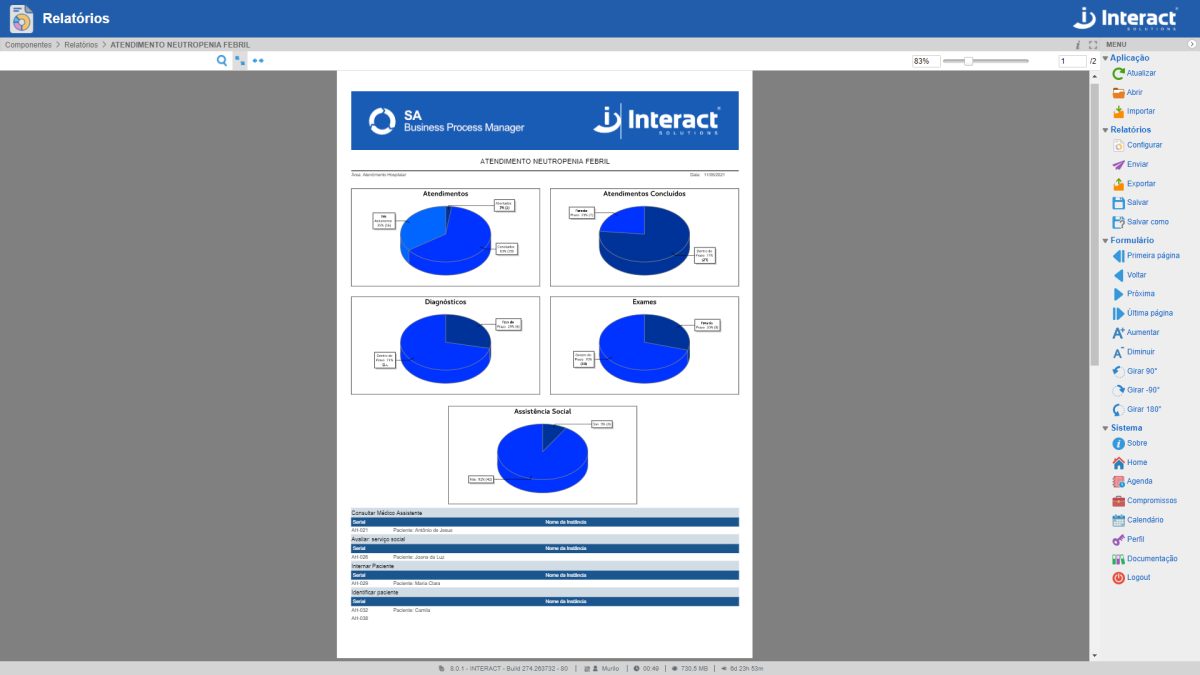
Reports
The Reports application makes it possible to generate reports with practically all the information contained in the system. The information can be customized, according to the user’s specific needs.


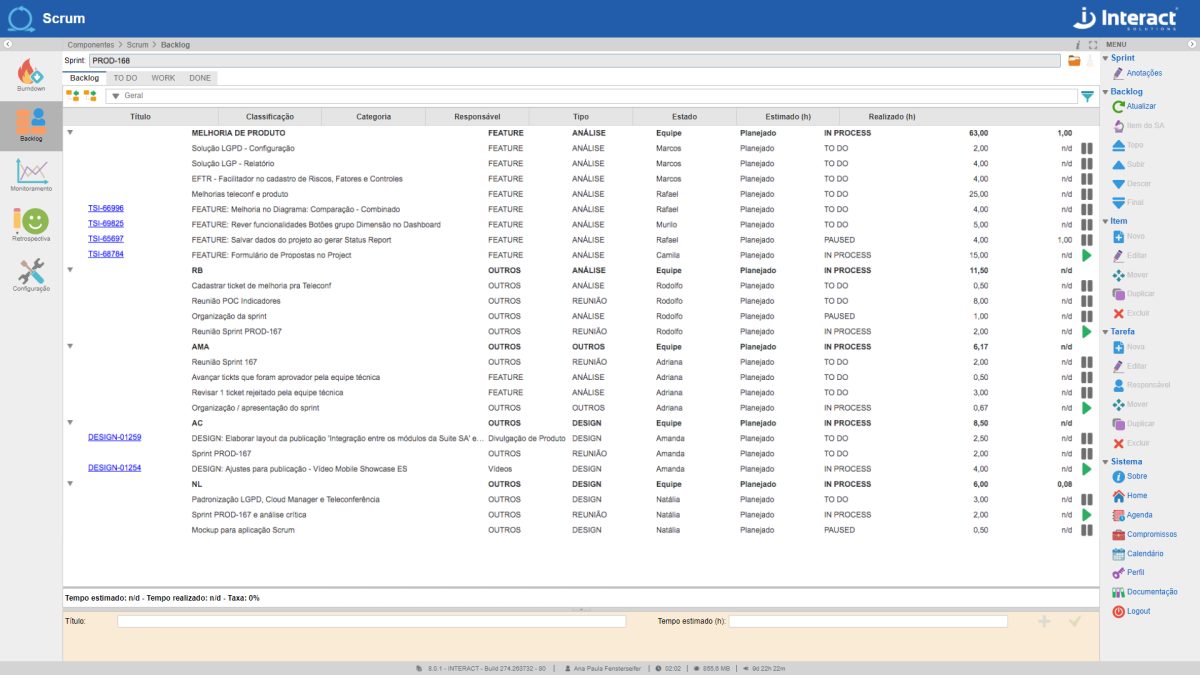
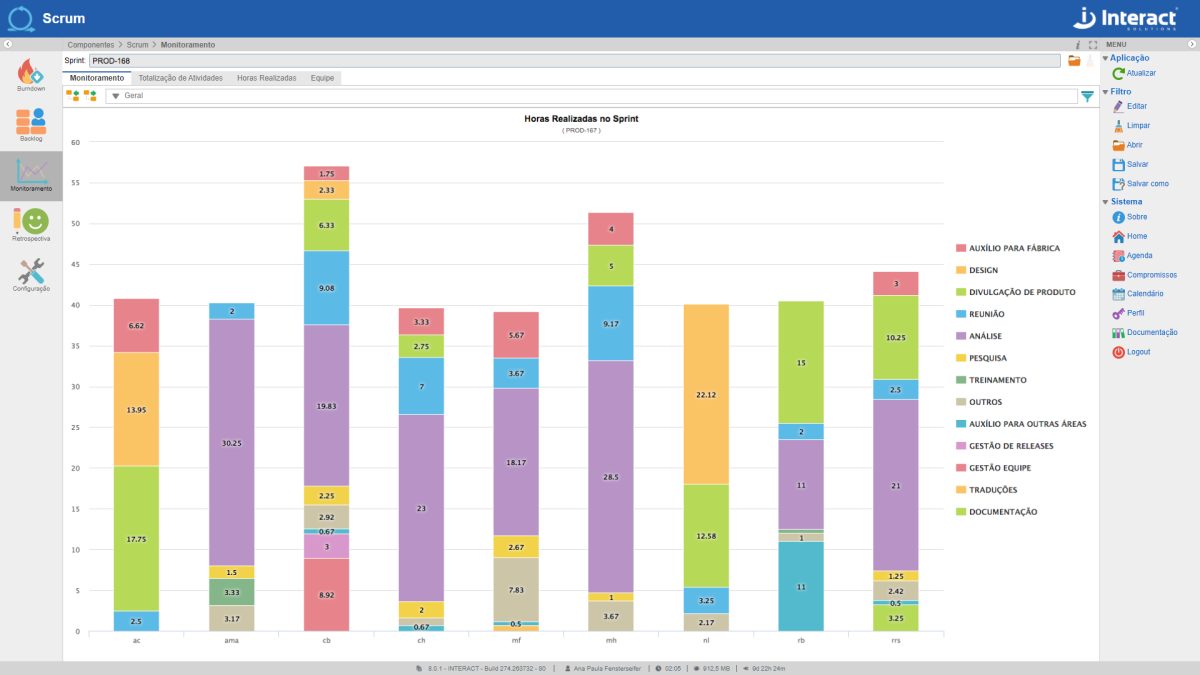
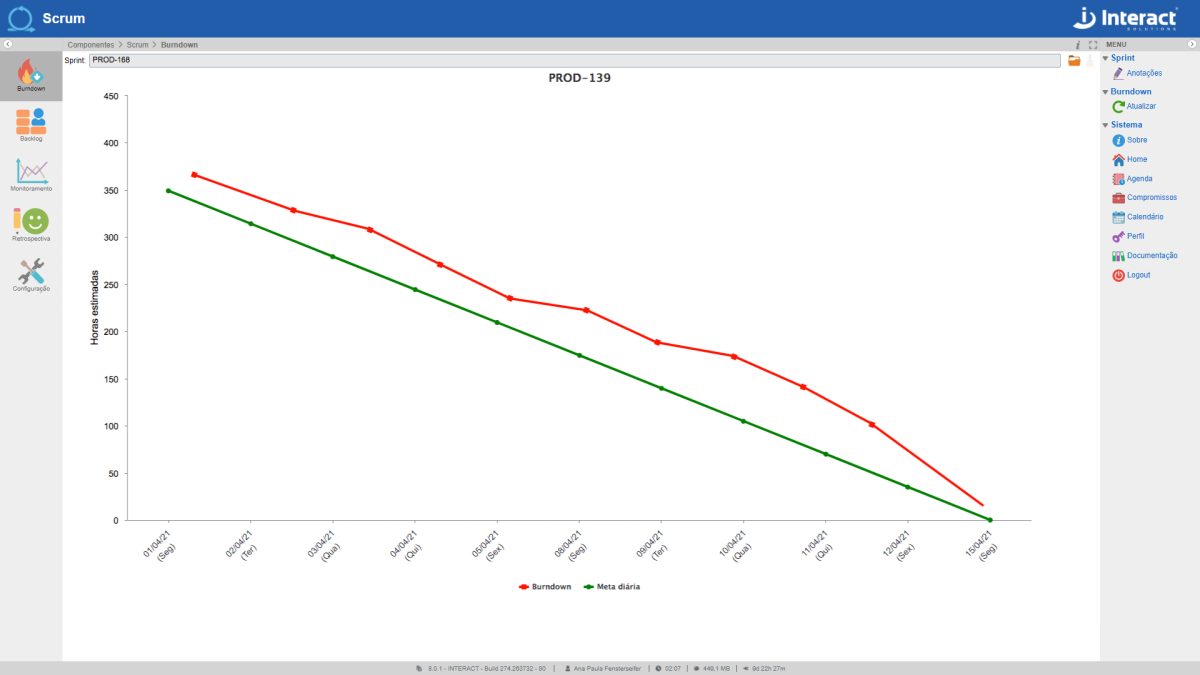
Scrum
One of the fastest growing methodologies in the world currently available to optimize the results of your company’s projects. Get to know Scrum, from Interact Suite SA, which guarantees the implementation of the agile Scrum methodology in your projects. Interact’s tool allows the creation of several project sprints and the monitoring of different teams.


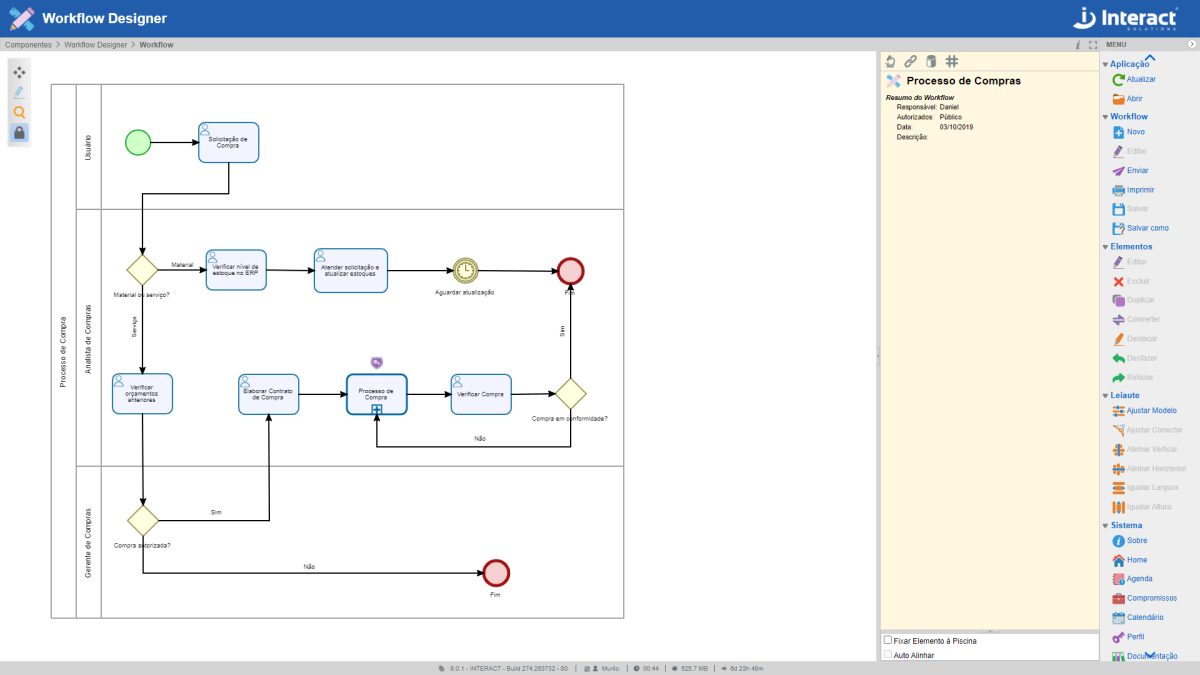
Workflow Designer
The Workflow Designer tool was developed to simulate and document processes or workflows. Similar to the elements of the BPMN (Business Process Model and Notation) notation, it is possible to model workflows according to a set of rules, with the aim of automating business processes. Integrated with the free tool, Interact Flow has current notation elements that meet modern methodologies.


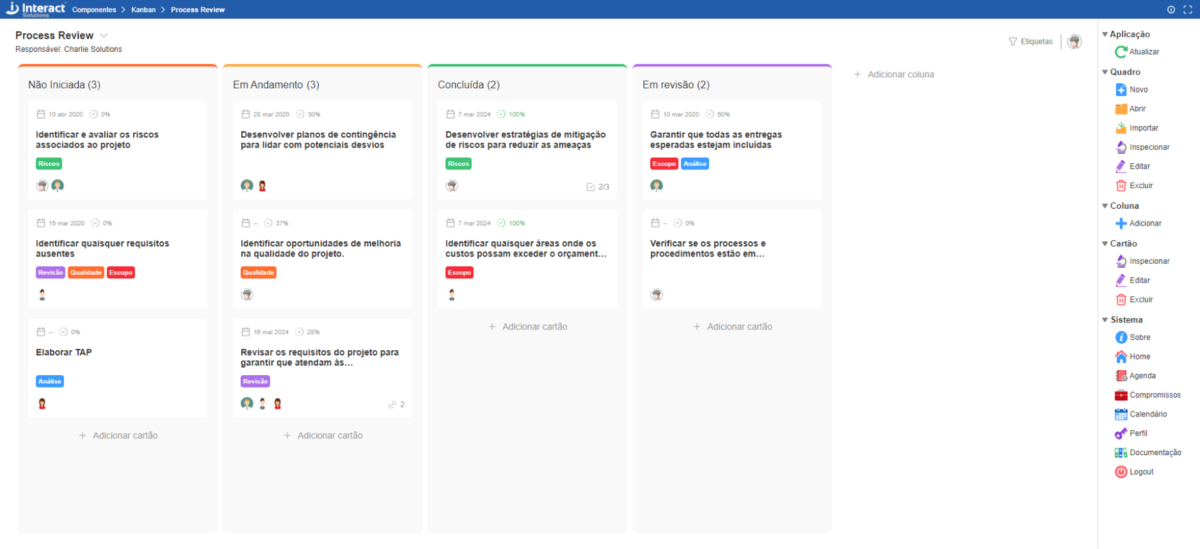
Kanban
Kanban stands out for providing agile and visual management of business operations. With this component, you’ll manage your team’s tasks in a simple and intuitive way. Just log the demands and organize them among the columns. You can sort and move them simply by dragging, and even add labels to categorize the demands. Take advantage of this new tool to enhance the management of your tasks and projects.


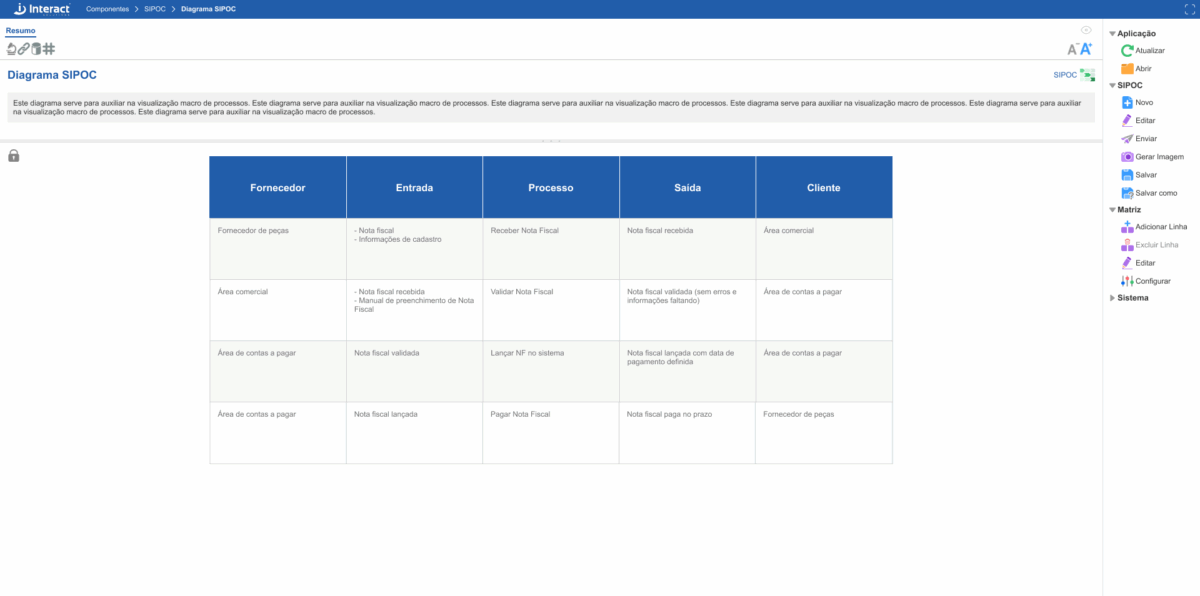
SIPOC
With the SIPOC tool, you can clearly and structurally map suppliers, inputs, processes, outputs, and customers, promoting a broad and detailed view of your operational flows. It is possible to customize some aspects of the diagram, such as the font type and the colors of the header and text. Additionally, you can adjust the height and width of rows and columns, ensuring that all information is visible.


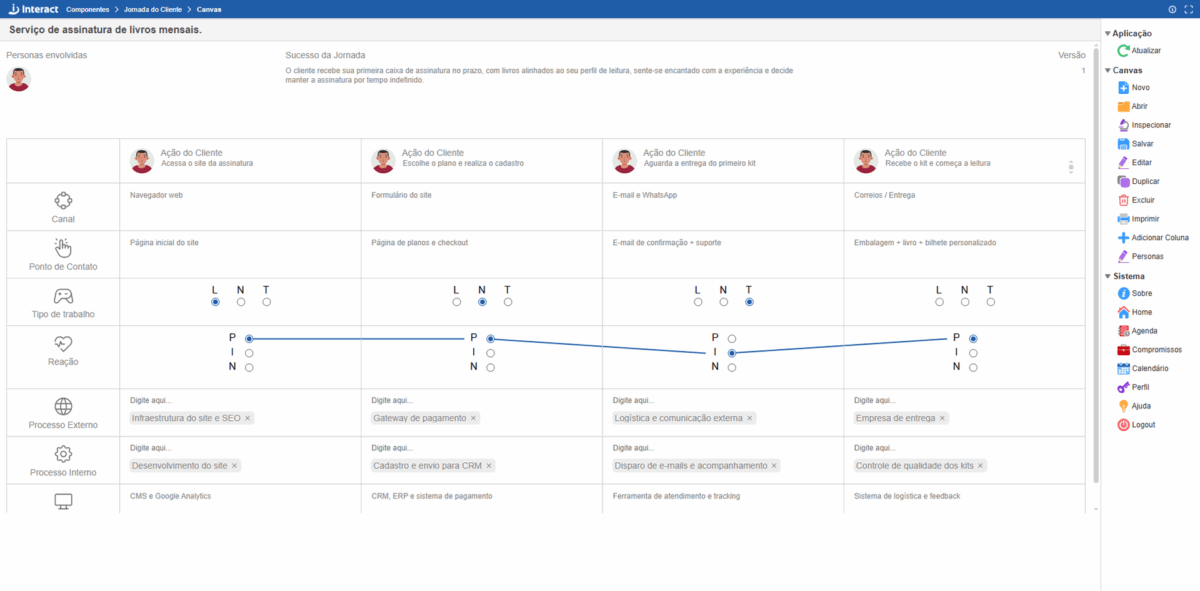
Jornada do Cliente
Método para entender com mais humanidade e relevância as necessidades de segmentos de mercado, desenvolver novas e poderosas ofertas de valor, projetar experiências positivas e memoráveis, conectar os processos organizacionais aos pontos de contato e momentos da verdade de cada experiência do cliente e, finalmente, orientar as lideranças organizacionais para a tomada de decisão mais estratégica sobre os clientes. Um guia essencial para entender clientes, desenvolver soluções, projetar experiências, repensar processos e prosperar.


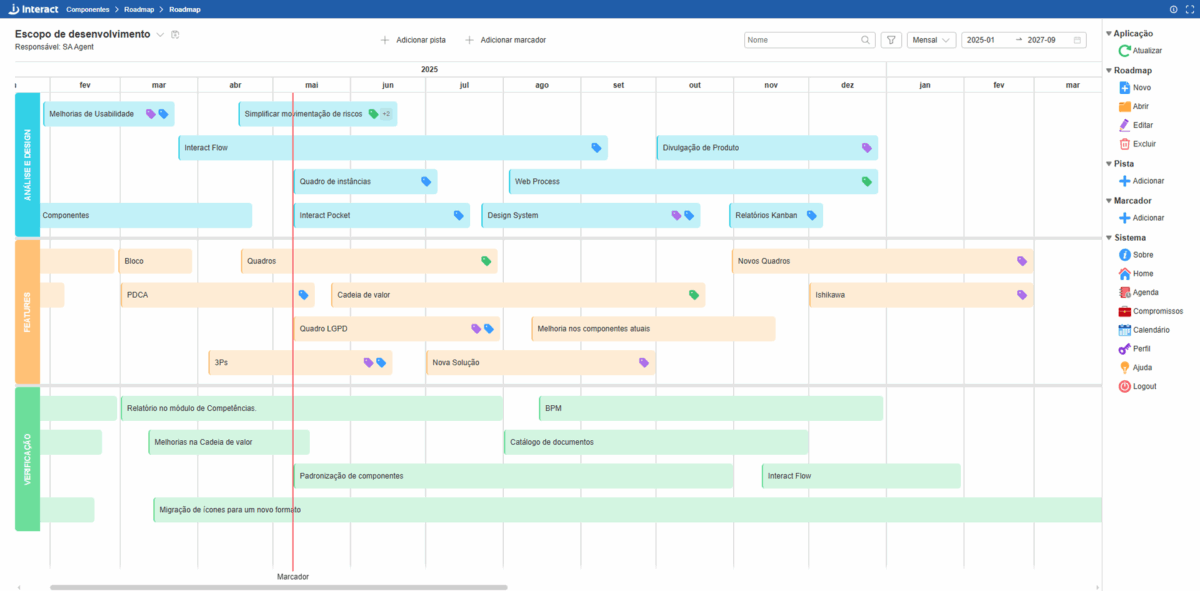
Roadmap
Turn your projects into reality. Create dynamic and customized roadmaps to guide your team. Monitor the progress of each stage and adapt to changes with agility.


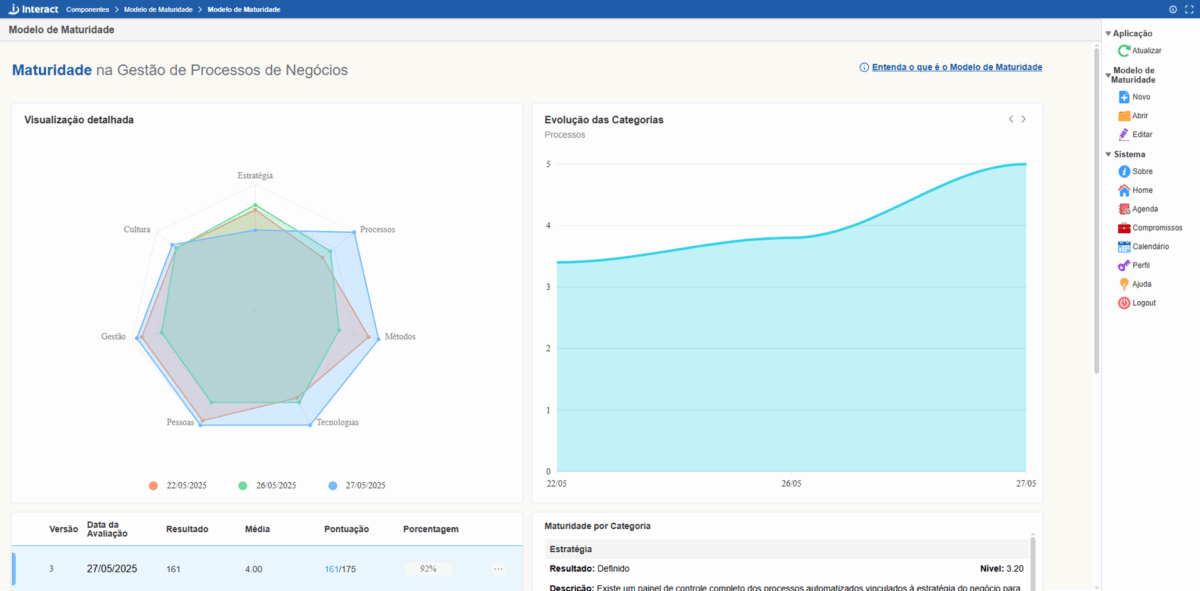
Maturity Model
The Maturity Model available in our Interact Flow product is now part of the Interact Suite SA component set. Inspired by the process management maturity model developed by Pedro Robledo, it features 7 fundamental and interconnected pillars for analyzing any organization.